Abstract
Introduction: Platelet aggregometry is one of the platelet function tests commonly used to assess platelet response to antithrombotics. Light transmission aggregometry (LTA) has long been considered the "gold standard" test, but has disadvantages, including the need to process blood into platelet-rich plasma to run the assay. Whole blood impedance aggregometry (IA) is another subtype of platelet aggregometry that avoids this processing, in which platelet binding to electrodes is used to measure aggregation. Prior studies have demonstrated nonconcordance of LTA and IA in measuring samples from patients on ticagrelor. A small-volume stenotic microfluidic is a potential novel platelet function testing tool that offers a more physiologically-fidelic platform for platelet aggregation with its ability to mimic flows and biological surfaces relevant to the vasculature. The device is made of collagen coated channels through which blood is withdrawn, mimicking post-plaque rupture and potentiating ex vivo thrombosis. The aim of this study was to compare a microfluidic assay in its ability to test antithrombotics with IA and LTA in the context of inhibition with ticagrelor and other antiplatelet agents.
Methods: Citrated whole blood from healthy volunteer donors (IRB21100141) was obtained and pooled. The blood was used to run the assays in parallel. Blood was incubated with different doses of ticagrelor for 5 minutes before each run. For LTA, blood was processed and platelet rich plasma was isolated. For both LTA and IA, the run lasted 10 minutes and the area under the aggregometry curve (AUC) was determined. Collagen (5 µg/mL) was used as an agonist to mimic in vivothrombotic occlusion, as also used in the microfluidic channel. For microfluidics, blood was stained with anti-CD41 to label platelets. Blood samples are added upstream and withdrawn via syringe pump (27 µL/min, initial arterial wall shear rate 3500 s-1). Fluorescent images were collected for 5 minutes and analyzed using MATLAB. Fold change in the mean fluorescent intensity (MFI) and area under the MFI curve (AUC) were determined.
Results:
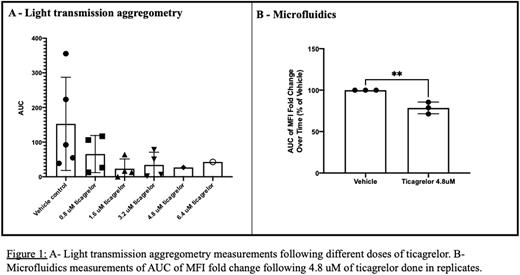
A reduction of platelet aggregation in all platforms was seen with increasing doses of ticagrelor (Figure 1-A). Impedance aggregometry AUC was reduced from 51.88 +/- 42.46 with vehicle control to 7.1 +/- 6.223 with 6.4 uM ticagrelor (reduction of around 86%). Light transmission aggregometry AUC was reduced from 152.9 +/- 134.5 with vehicle control to 42.90 with 6.4 uM ticagrelor (reduction of around 72%). Microfluidics AUC was reduced by 74% of that of the vehicle with 6.4 uM of ticagrelor. The variability noted in microfluidics was far less pronounced than that seen in platelet aggregometry (Figure 1-B).
Conclusion: A small-volume stenotic microfluidic device offers a potential alternative to platelet aggregometry for testing of platelet function after use of antithrombotics. This assay presented advantages such as the use of small sample volumes (~200 µL), no need to further process samples as required in LTA, and having less variability between replicates, in addition to the fact that it represents a physiologic understanding of platelet aggregation and clot formation. This platform will allow further mechanistic exploration and further study of other known and novel platelet inhibitors.
Disclosures
Neal:Haima therapeutics: Other: Scientific advisory board; Janssen: Research Funding; Haemonetics: Research Funding; Instrumentation Laboratory: Research Funding; Meredian: Honoraria; Haemonetics: Honoraria; CSL Behring: Honoraria; DoD: Research Funding; NIH: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal